Abstract
Introduction: Patients with polycythemia vera (PV) have a high symptom burden (eg, fatigue, pruritus, and splenomegaly-related symptoms), an increased risk of thrombotic events, and the potential for transformation to myelofibrosis or acute myeloid leukemia. Control of peripheral blood counts is a primary treatment goal to reduce the risk of PV-related complications. The current analysis of the REVEAL study aims to compare the symptom burden between patients with PV who achieve blood count control and those who do not.
Methods: The ongoing REVEAL study (ClinicalTrials.gov, NCT02252159) is a prospective, multicenter, observational study of adult patients with PV in the United States. During the 36-month observation period, clinical data are being collected from usual care visits. Symptoms are assessed using patient-reported outcome questionnaires at enrollment and every 3 months thereafter. Patients included in this analysis were required to have a complete blood count (CBC) result within 30 days of completing the at-enrollment Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF). Total symptom score (TSS) is calculated as the sum of the individual symptom scores in the MPN-SAF. Complete hematologic response (CHR) is defined as achieving hematocrit (HCT) ≤ 45%, white blood cell (WBC) count ≤ 10 × 109/L, and platelet (PLT) count ≤ 400 × 109/L. Patients who did not achieve control in at least 1 of the counts (ie, HCT > 45%, WBC count > 10 × 109/L, or PLT count > 400 × 109/L) are categorized as not achieving a CHR (non-CHR). In addition to TSS, the 3 most commonly reported PV symptoms are summarized. Data cutoff for this analysis was May 18, 2017.
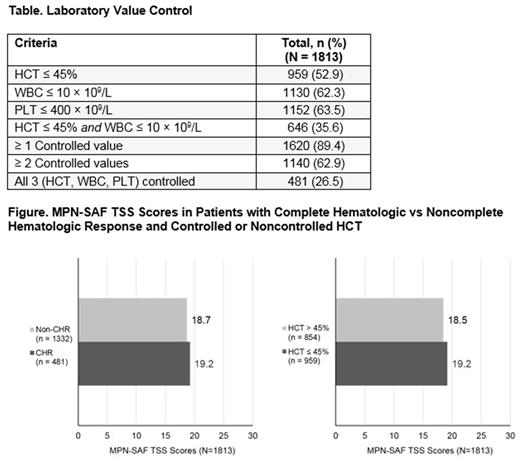
Results: Of 2510 patients enrolled in REVEAL, 2307 completed the MPN-SAF at enrollment; of those, 1813 had a CBC available within 30 days of enrollment. Among evaluable patients (n = 1813), 54.3% were male, 90.6% were white, and the median age was 67 years (range, 22-95 years); 481 patients (26.5%) had a CHR at enrollment, and 959 (52.9%) had an HCT ≤ 45%. Other counts are presented in the Table. Mean TSS scores were similar among CHR and non-CHR cohorts (19.2 vs 18.7, respectively) and among patients with HCT levels ≤ 45% or > 45% (19.2 vs 18.5, respectively), as illustrated in the Figure. Furthermore, the severity of the 3 most commonly reported symptoms (fatigue, early satiety, and inactivity) was similar whether patients were in CHR or not. These same trends in symptom severity were observed when other laboratory parameters (eg, HCT, WBC, PLT) were assessed individually.
Conclusions: Approximately half of evaluable patients had achieved HCT ≤ 45% and 26.5% had achieved CHR based on controlled HCT, WBC, and PLT counts at the time of enrollment. Patients reported similar symptom severity regardless of whether their HCT was ≤ 45% or whether other blood counts (WBC and PLT) were controlled, suggesting that patients with controlled blood counts may continue to experience PV-related symptoms. The current management of patients with PV is largely focused on blood count control, with the aim of reducing the risk of PV-related complications. These findings highlight the importance of regular symptom assessment during the usual care of patients with PV.
Grunwald: Cardinal Health: Consultancy; Janssen: Research Funding; Pfizer: Consultancy; Celgene: Consultancy; Incyte: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; ARIAD: Consultancy; Forma Therapeutics: Research Funding; Genentech: Research Funding; Alexion: Consultancy. Burke: Gilead: Consultancy; Genentech: Consultancy; Incyte: Consultancy; Celgene: Consultancy; Bayer: Consultancy. Kuter: Merck: Consultancy; Protalex: Consultancy, Research Funding; Incyte: Consultancy, Research Funding; Shire: Consultancy, Research Funding; 3SBIO: Consultancy; ONO: Consultancy; Dova: Consultancy, Membership on an entity's Board of Directors or advisory committees; Argenx: Consultancy; Fujifilm: Consultancy; Zafgen: Consultancy; Genzyme: Consultancy; Pfizer: Consultancy; Amgen: Consultancy; Alexion: Consultancy, Research Funding; Rigel: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Syntimmune: Consultancy, Research Funding; Novartis: Consultancy. Gerds: Incyte: Consultancy; CTI BioPharma: Consultancy. Stein: Incyte: Consultancy. Savona: Astex: Membership on an entity's Board of Directors or advisory committees, Research Funding; Karyopharm: Consultancy, Equity Ownership; Sunesis: Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees; Takeda: Research Funding; Gilead: Membership on an entity's Board of Directors or advisory committees; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees; Incyte Corporation: Consultancy, Research Funding. Naim: Incyte Corporation: Employment, Equity Ownership. Colucci: Incyte Corporation: Employment, Equity Ownership. Paranagama: Incyte Corporation: Employment, Equity Ownership. Mesa: Ariad: Consultancy; Promedico: Research Funding; Novartis Pharmaceuticals Corporation: Consultancy; Gilead Sciences, Inc.: Research Funding; Galena Biopharma, Inc.: Consultancy; Celgene Corporation: Research Funding; Incyte Corporation: Research Funding; CTI BioPharma Corp.: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal